Nanodiamond Particles
Nano-particles of diamond have many applications when they are sufficiently dispersed in a solution. The production of mono-disperse colloids of diamond or carbon nano-particles is a science in itself, which requires a fundamental understanding of the interaction of the particle surfaces with ion / molecules in the solvent. At Cardiff Diamond Foundry we purify commercially available material (mostly detonation nanodiamond) as well as produce our own diamond nanoparticles from scratch (for quantum photonics applications)
Detonation Nanodiamond purification and self assembly
The smallest diamond nano-particles are produced by detonation synthesis. This process relies on the fact that during an explosion, the pressure and temperature are just right for diamond growth. Unfortunately this only lasts for around a micro-second so the particles to grow to around 5 nano-metres. During the cooling cycle of the explosion the pressure and temperature drop and non diamond carbon is also grown. This material glues together these diamond particles into larger aggregates which are very difficult to break down.
We have developed procedures to break down these particles down to into their minimum sizes by annealing them in hydrogen (full details here). These particles exhibit strong positive charges in water (zeta potential) which stops them from agglomerating over long periods of time. This charge can be controlled by modifying the atoms/molecules at the surface of the particles. The strong positive charge is related to non diamond carbon at the surface and the reduction of oxygen species which otherwise would render a strong negative charge (see full explanation here). This charge is also sensitive to pH, providing another mechanism for control of surface charge. The control of this charge can be used to determine whether particles adhere to a surface or not, in the images below we show how we can drive self assembly of very high densities of particle on various surfaces.
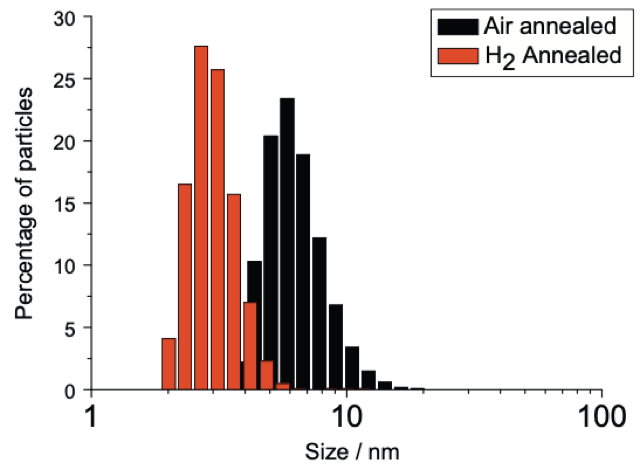
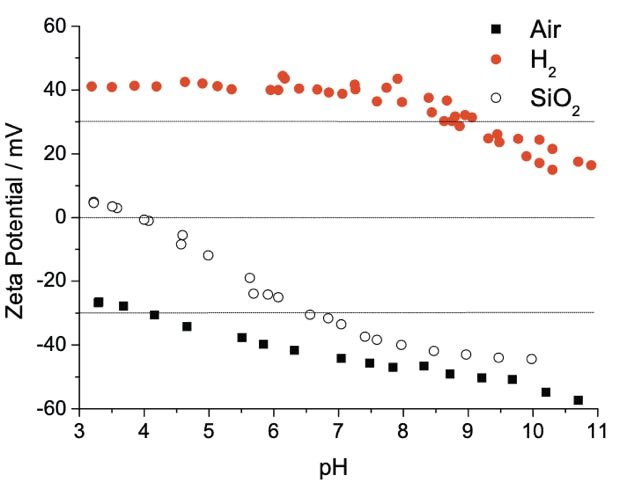
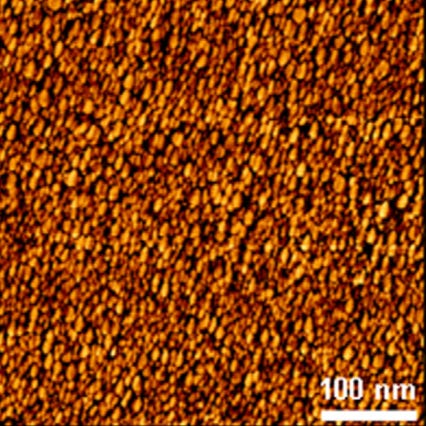
Left to right: Dynamic Light Scattering (DLS) data showing mono disperse size distribution of hydrogen and oxygen annealed detonation nanodiamond; Zeta potential titrations vs pH of hydrogen and oxygen annealed detonation nanodiamond as well as silicon dioxide; Atomic Force Microscopy image of hydrogen annealed detonation nanodiamond particles on silicon with density approximately 1012 cm-2. This is how we seed diamond growth on most surfaces, we have shown similar densities on AlN, SiN and GaN, see:
Williams et al, CPL 509 (2011) 12 (Silicon)
Hees et al, Nanotechnology 24 (2013) 025601 and Mandal et al, ACS Materials & Interfaces 11 (2019) 40826 (AlN)
Mandal et al, ACS Omega 2 (2017) 7275 (GaN)
Bland et al, Scientific Reports 9 (2019) 2911 (SiN)
Production of high purity nanodiamonds with custom colour centres
We have developed procedures to produce ultra high purity nanodiamond particles by milling diamond produced by Chemical Vapour Deposition. This also allows us to produce nanodiamonds with custom impurities such as nitrogen or silicon for the Nitrogen Vacancy and Silicon Vacancy single phon centres. The process involves growth of either high purity diamond with additional nitrogen/silane, high energy milling into nano sized particles followed by extensive purification. This process is shown below (full details here):

CVD Diamond growth with solid silicon source, silane, nitrogen etc
High energy planetary milling
Purification and size selection
A key issue with the milling process is the contamination introduced. As diamond has a Moh’s hardness of 10, any material used to mill the diamond will also be damaged and incorporated into the resulting powder. Traditionally, diamond is milled with steel, leaving very low concentrations of iron / iron oxide behind (see below). For the majority of applications this is no issue, but for magnetometry applications of the NV centre in diamond this is problematic. We have developed a metal free milling process to avoid this problem. The material still requires substantial purification post - milling such as acid reflux and high G centrifuge removal of large particles.
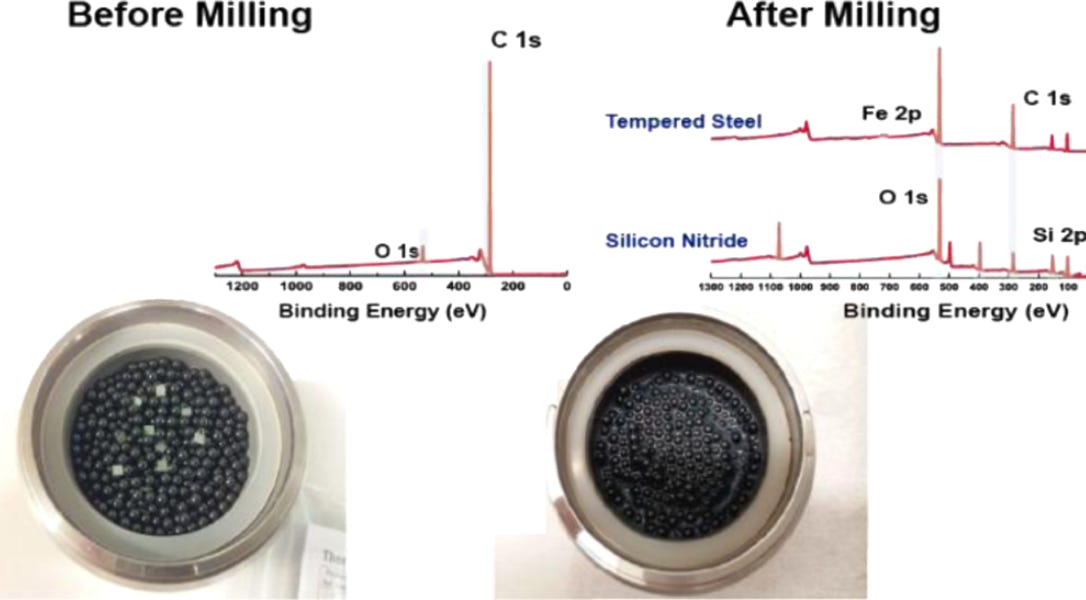
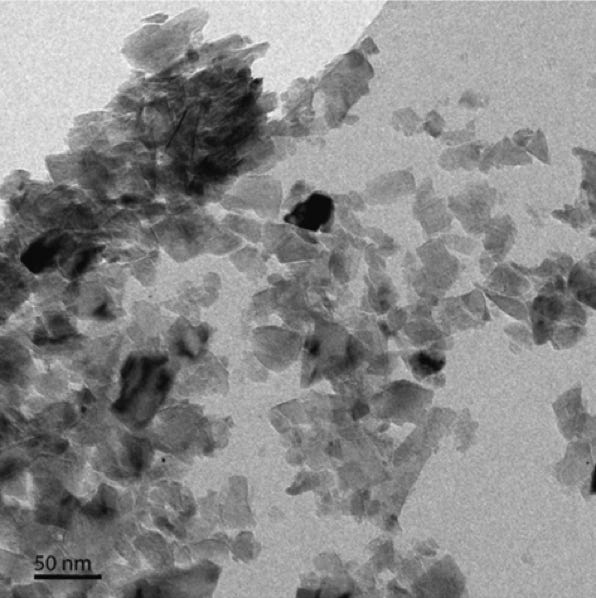
Left to right: Diamonds before milling sitting on top of the steel (or SiN) bearings with XPS survey scan (above inset); after milling the resulting powder is dark due to non-diamond carbon content that needs to be removed. In the XPS survey scan Fe and Si contaminants are clearly visible. This is after significant acid reflux and other post cleaning processes. Far right shows TEM of the resulting particles, the mean particle size is around 50 nm, we are able to produce down to about 20-30 nm as confirmed by Dynamic Light Scattering (below). See:
Gines et al, ACS Omega 3 (2018) 16099
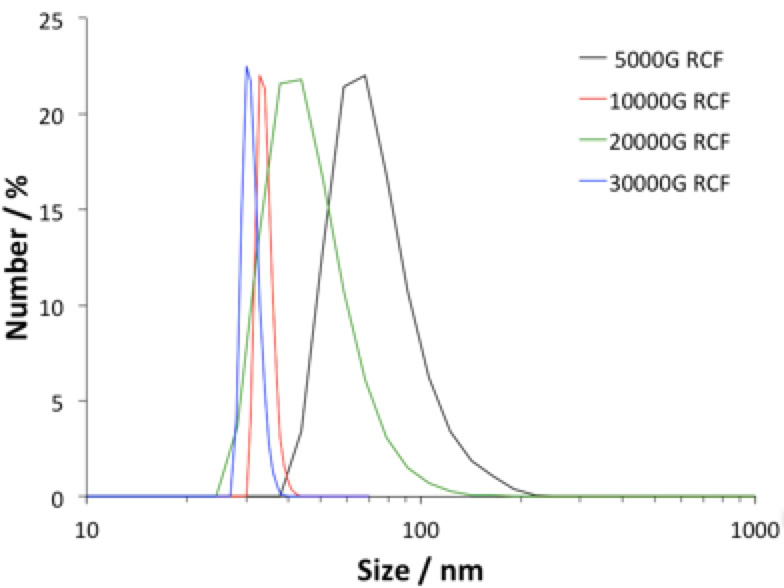
Left: Particle size distribution after removing larger particles and debris with high G centrifugal force (up to 30K RCF).
Recently, in collaboration with the Morley group at the University of Warwick, electron spin coherence (T2) times as long as 400 µs at room temperature have been demonstrated from Element Six materials milled by this approach. This is significantly higher than in common commercially available material. See: